By Adam Thoms, Ph.D., and Nick Christians, Ph.D.
The soil from which sports fields are constructed often present some difficult problems for the field manager. Contractors often give little thought to the soil during the construction process, and the result can be very difficult to deal with for years to come. A good example of this are the heavy clays from which many fields are constructed. Many of our newer fields are constructed from a media that is mostly sand. While this improves drainage, it presents some other problems from a nutritional standpoint. Every field is going to be different. In the same job, you may be managing some fields that are sand, some that are constructed of a clay-loam soil, and some with very poor soil that is mostly clay.
The best way to deal with these varying conditions is to arm yourself with a basic knowledge of the soil and how it functions. In this article, we will cover the principles of soil science and provide you with some information that can help with the management of a variety of soil conditions.
There are many commercial laboratories that provide soil testing services. Some are good and some are not. It is important that you familiarize yourself with the soil testing services available in your region and find those with a good reputation for providing accurate and useful information for turfgrass managers. Companies that sell fertilizers and other turfgrass products will sometimes offer soil testing at no cost. While these can be useful, it is always risky to get all of your soil test information from those who are selling you products. It is best to learn some of the basics of soil testing and provide your own interpretations of the soil test data from an independent lab. There is no one better than the on-site manager to determine what is really needed for the fields that they manage. For a more in-depth discussion of soil testing, see “Fundamentals of Turfgrass Management” (Christians, et. al, 2017)
Interpretation is the key to a good soil test. Studies have shown that as long as a similar testing method is followed, soil-testing laboratories are generally quite consistent in the test numbers that they generate. It is the interpretation of what is needed based on these tests that can vary widely.
Let’s begin with the information that will be presented on good soil test reporting form. The first line on many forms is listed as CEC. This abbreviation is followed by a number. The CEC stands for “Cation Exchange Capacity.” The cations are a series of positively charged (+) ions that play important roles in plant nutrition. They include such elements as Hydrogen (H+), Calcium (Ca++), Magnesium (Mg++), Potassium (K+), and Sodium (Na+). Note that some are monovalent (have one positive charge) and some are divalent (have two positive charges). Whether an element is monovalent or divalent has some important ramifications for its use.
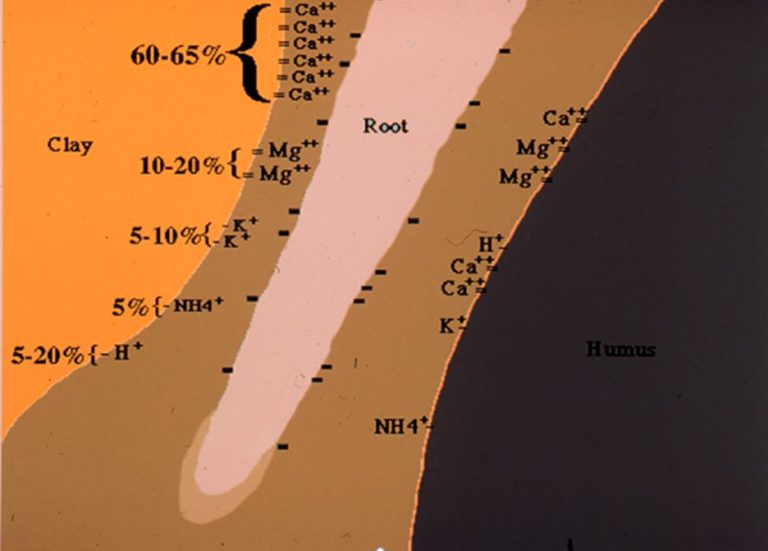
The soil has a series of negative (-) charges on fine soil particles and on the organic materials from which it is formed. The + and – charges are like magnets. Positives repel positive charges and negative repel negative charges; however, when a + charge gets close to a – charge, they bond like magnets and the cations are held in place on the particles for exchange with the root system (Figure 1, above). The more negative charges a soil has, the more cations it can hold in place for the plant to use. The CEC is listed in millequivalents/100 g of soil (meq/100g). We won’t go into details on how this number is derived, but even 1 meq/100g is many billions of – sites. These are very small and it takes a lot to supply enough nutrition to the plant. The higher the number, the more of these sites are available to hold cations in place.
Sand have very low CEC, often 1 or less, whereas clays have a much higher CEC, as much as 80. While clays have a high CEC, they generally have poor physical characteristics and do not provide a good soil media for sports fields. The very low numbers in a sand can be insufficient to supply enough of some elements for plant growth. We would generally like to see a CEC of 25 to 30. The clay-loam soils of the Midwestern prairie will generally be in this range, and they have enough CEC sites to hold nutrients in place and have satisfactory physical characteristics for plant growth.
The next line on a soil test will generally be pH, written with a small p and a big H. The H stands for hydrogen (H) and this test is a measure of how much H is in the soil system. While H is not taken up directly by the root system, its concentration in the soil is very important. If there is an H on the cation exchange site, by default there is not a Ca or a Mg, or a K. The pH tells a lot about what is on CEC sites in the soil (Figure 2).
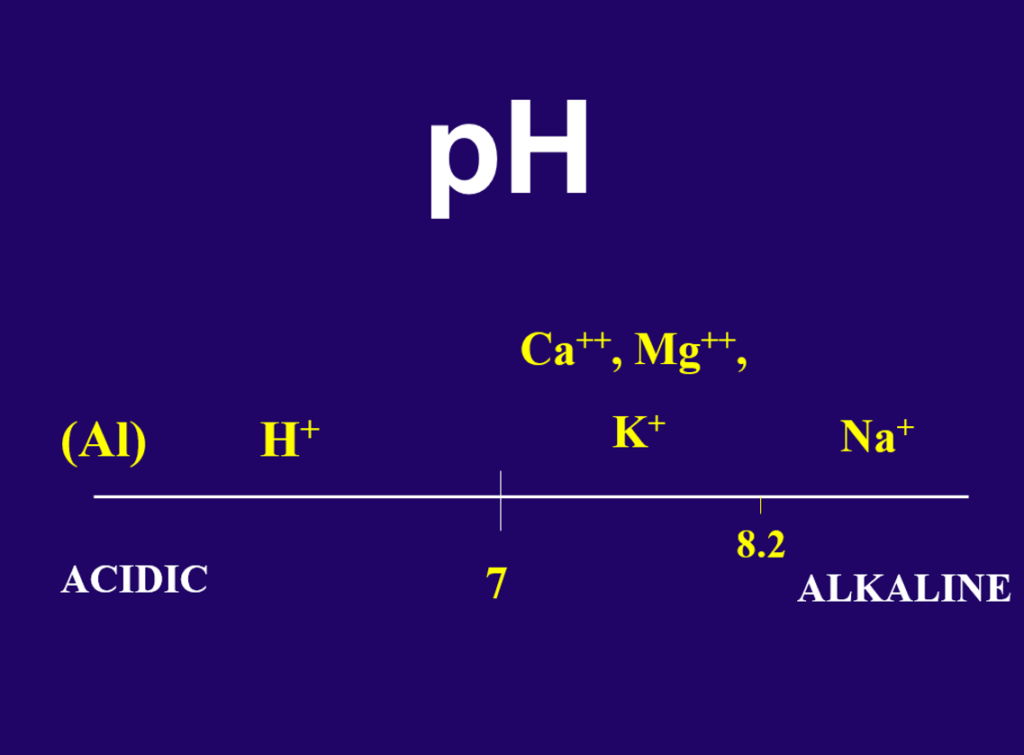
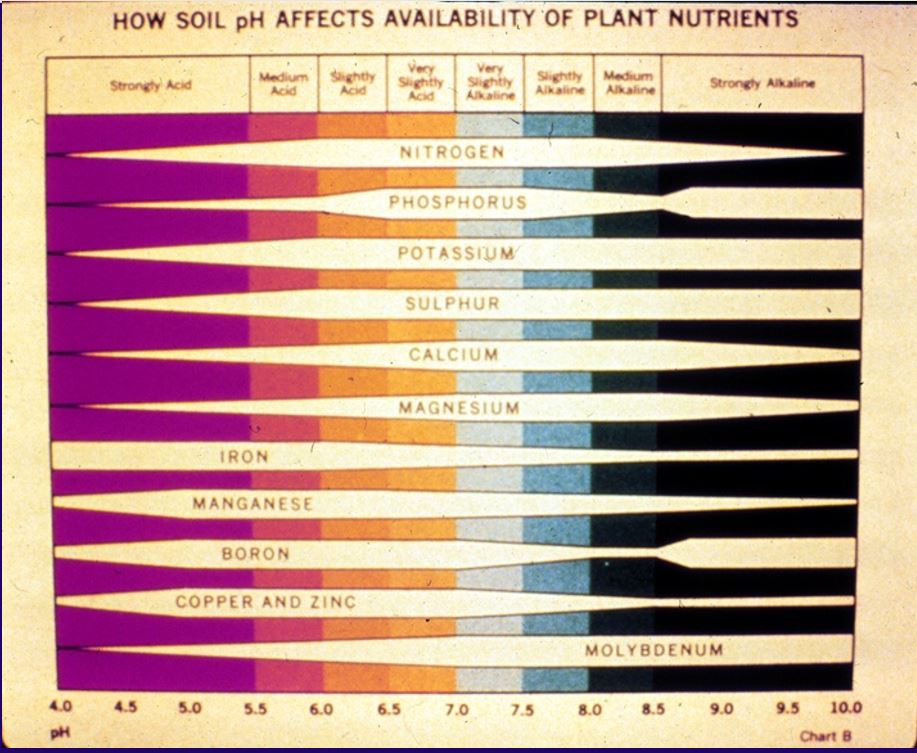
The pH scale ranges for 1 to 14 with 7 being the neutral point. The pH scale is an inverse scale meaning the higher the number, the less H is in the soil and the lower the number, the more H is present. Soils with pH values below 7 are referred to as “acidic.” This means that there is a lot of H in the soil system and on the CEC sites. It would be expected that these soils would be deficient in elements like Ca, Mg and K. The soils with pH values above 7 are called “alkaline.” Alkaline soils will likely have sufficient amounts of the Ca, Mg and K, but they may be deficient in elements like iron (Fe) (Figure 3). Plants grown on these soils may show signs of yellowing (iron chlorosis). We would like to see the soil in the range of 6 to 7. This pH generally has the best balance of available nutrients for plant growth. This does not mean that you cannot grow grass on soils with acidic or alkaline pH values, but modifications to the fertility program may become necessary. Most common is the addition of iron in the fertility program of turfgrass grown on high pH soils.
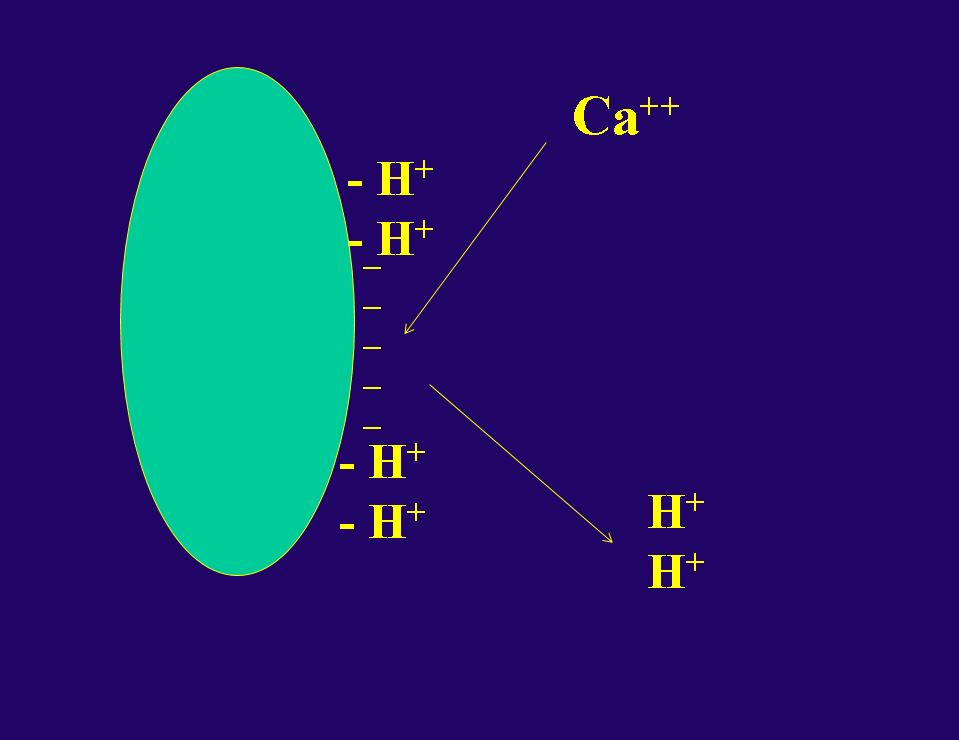
If the soil has an excessively low pH, the solution will be to lime the soil. Lime is CaCO3 and it is a common additive on low pH soils. The lime raises pH by replacing H on the CEC sites with Ca (Figure 4). This process is slow, and it may take two or more seasons to raise the pH to satisfactory levels. During the liming process, it is important to consider the balance of the other elements on the CEC sites, and some Mg and K may also play an important role in modifying the soil.
The next line on the soil test will likely be buffer pH. This is a separate test that is conducted to determine how much lime is needed to raise the pH of a given soil. The amount of lime required can vary by several tons per acre and a buffer pH test should be conducted to determine the amount of lime needed on your particular site. The test is only run if the pH of the soil is below 6. There is no reason to run it if no lime is required. The buffer pH will be presented as a number. To interpret this number in lbs. of lime/acre, a buffer pH table it required. These tables can be found online, or you can refer to page 153 in the 5th edition of “Fundaments of Turfgrass Management” (Christians et al., 2017).
If the pH of the soil is excessively high, 7.5 to 8.2, the solution may be acidification. This can sometimes be done by adding sulfur, which forms H2SO4 (sulfuric acid) in the soil solution. This provides H+ that is attracted to the CEC sites and thereby lowers the pH. The problem is that many of our high-pH soils are highly buffered, meaning that they resist pH change and acidification. This is particularly true of soils with excess levels of calcium carbonate in them. These soils have pH values of 8.2 and are nearly impossible to acidify. On these soils, it is better to adjust the fertility program by adding needed elements, such as Fe, rather than trying to lower the pH.
The remainder of the soil test form will give you an evaluation of how much of each of the essential elements are available for plant use. The best tests will give you an estimate of the percentage of the cations on the cation exchange sites and an evaluation of the parts per million (ppm) or pounds per acre (lb/a) available for the major elements like phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg) and a selection of the micronutrients. The key to understanding this is knowing how to properly interpret these numbers.
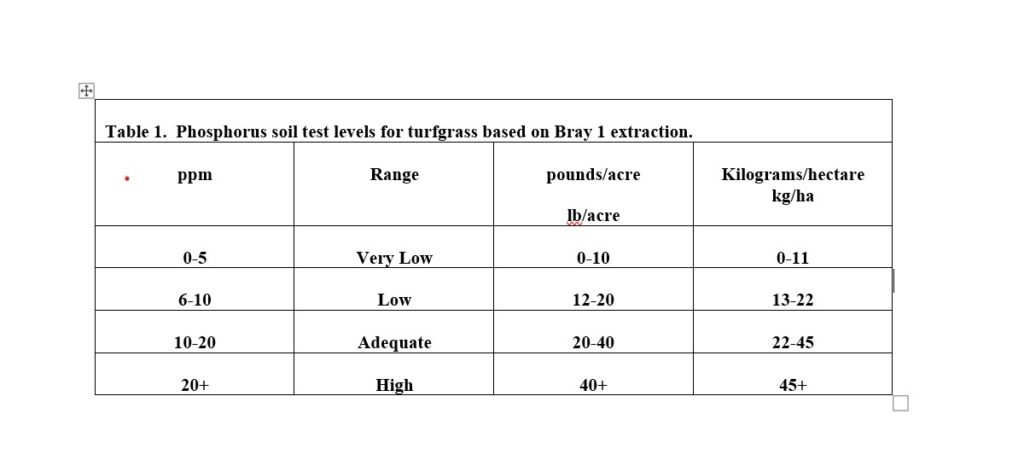
Phosphorous is one of the most important nutrients for turfgrass, especially during establishment and when a plant is developing a root system. In the soil, phosphorous is immobile, which means the plant must come in contact with nutrient to be taken up. Starter fertilizers often will contain levels of phosphorous that are twice that of the nitrogen level, while maintenance fertilizers often have much lower levels of phosphorous due to the turfgrass having an extensive fibrous root system that can mine nutrients from the soil. Soil tests will extract plant-available phosphorus. Depending on what soil extraction method was used, will depend on what values will be given for the amount of phosphorus present. An example is the Bray 1 method, which says 10-20 ppm is adequate for turfgrass growth (Table 1). Many other crops are annual, and would require higher amounts of phosphorous, so make sure you are following recommendations for turfgrass growth.
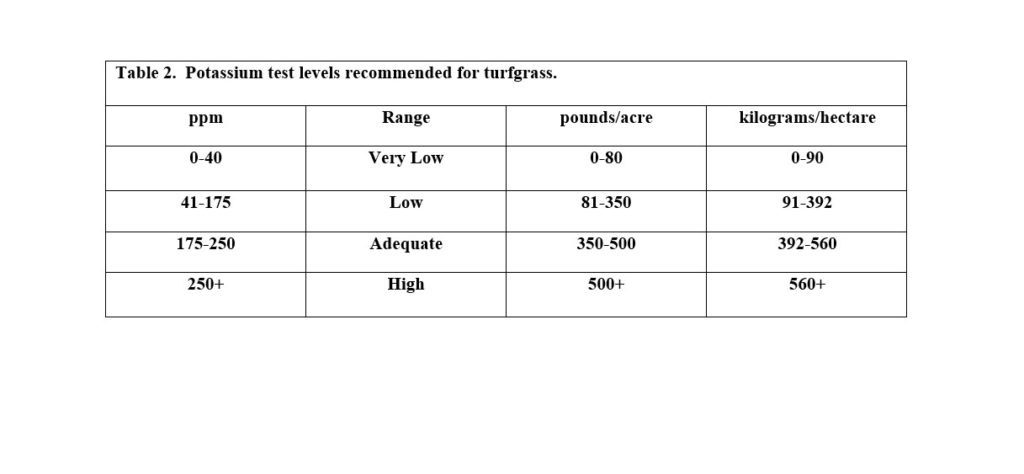
Potassium can help with plant growth as well as stress tolerance. In fact, potassium is often applied in excess of that needed for plant yield, as higher levels of potassium can help with stresses such as traffic. Applications of potassium are very common with fertilizers like 12-3-12, 14-7-14, and 22-0-10 for example. Since stress tolerance is hard to quantify, regular applications may be necessary, but often 175-250 ppm is considered adequate levels for turfgrass (Table 2). Sand-based root zones may need regular applications as there may not be enough CEC sites for these kinds of levels. Never apply more than one pound of a nutrient per 1,000 ft2 to turfgrass foliage to avoid injuring the turfgrass.
Magnesium is often deficient when soil pH is low and there is a low-CEC soil. The basic cation saturation ratio (BSCR) will often underestimate the magnesium in the soil. Test applications to a small area to watch for a response. This will tell you if a larger application is warranted. Iron soil tests are also often unreliable, and iron should be applied with small test areas to watch for a response before large-scale applications are made. We will not talk about calcium in this article as we have covered in extensively in a previous article two years ago (Christians and Thoms, 2020). Micronutrients were also covered in detail in 2017, and will not be covered here (Christians and Thoms, 2017).
Soil testing can be a great help in directing what nutrients are present, but interpretation of those values is what can make a great difference in what you need to apply. Mismanagement of these applications can lead to poor plant performance and overspending of budgets. Knowing what to apply, and when, often makes for successful turfgrass managers and successful fields.
Adam Thoms, Ph.D., is assistant professor specializing in commercial turfgrass management, Iowa State University, Department of Horticulture, Ames, Iowa. Nick Christians, Ph.D., is University professor of turfgrass management, Iowa State University, Department of Horticulture, Ames, Iowa.
References:
Christians, N., and A. Thoms. 2020. “So, What’s the Deal with Calcium.” SportsField Management. Feb. 36(2):32, 34-35.
Christians, N., and A. Thoms. 2017. “How about the Micronutrients?” SportsTurf. Feb. 33(2):16-18.
Christians, N.E., A.J. Patton, and Q.D. Law. 2017. “Fundamentals of Turfgrass Management,” 5th ed. Wiley & Sons, Inc. Hoboken, NJ.
Troug, E. 1946. “Soil reaction influence on availability of plant nutrients.” Soil Sci. Amer. Proc. 11:305–308.