By Nick Christians, Ph.D., and Adam Thoms, Ph.D.
At Iowa State University, we get several contacts each week seeking information on all areas of turfgrass management. We often see patterns in these requests that tell us a lot about what the members of our industry are thinking. One of these patterns in recent years has centered on the use of calcium in sports field and golf management. The conversation generally begins with, “Someone wants to sell me a calcium supplement for my turf, do I need it?” This question does not have an easy answer. It is one of those things that has so many variables surrounding it that more information is needed – followed by a detailed discussion. So, what is the deal with calcium?
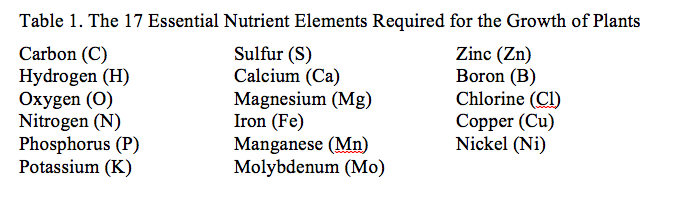
Calcium is an element, one of the basic building blocks of living systems. It is one of only 17 elements that are deemed to be essential for all plants (Table 1). While nitrogen (N), phosphorus (P) and potassium (K) generally are the elements that get the most attention, calcium (Ca) plays a number of important roles in a sound turfgrass field management program. You are probably familiar with the terms macronutrient and micronutrient. Most people are surprised to find that Ca is a macronutrient according to the strict definitions of plant physiology. A macronutrient is found in the plant in quantities of 1,000 parts per million (ppm) or more, and a micronutrient is found in quantities of 100 ppm or less. By this definition, Ca is a macronutrient.
Calcium plays a number of very important roles in the plant. It is involved in cell wall formation, which can be related to wear tolerance, an important consideration for both sports and golf turfgrass management. It is involved in cell division, membrane stability, osmotic balance and several other important functions related to growth and stress tolerance. While the deficiency symptoms observed on the plant for many of the other elements, like N and iron (Fe), is a yellowing of the plant, termed chlorosis, this is not the case for plants lacking enough Ca. True, visible deficiency symptoms of Ca on grasses are rare; but when it happens, it appears as a reddish discoloration of the younger leaves. For reasons that will be discussed, this generally appears in soils with low cation exchange capacity (CEC) and low pH.
While these can occur in turfgrass areas, it is much more common to have neutral to higher pH values in most turfgrass areas and true Ca deficiency on turfgrass is rare.
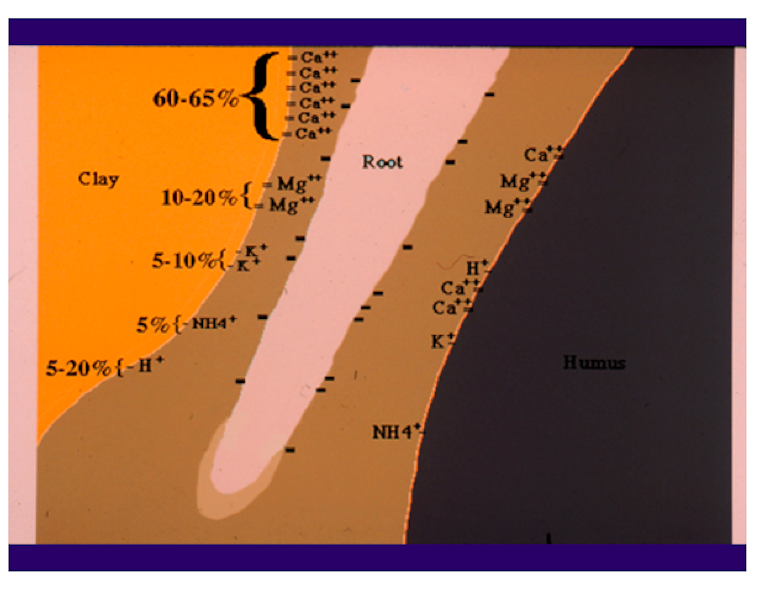
Calcium is a cation. It has two positive charges and is often written as Ca++. The positive charges of the Ca and similar cations are attracted to the negative charges on soil particles and, in a similar fashion to unlike poles of magnets, are held in place for exchange with the root system (Fig. 1). Rootzones vary in CEC and soils, like sands, with low CEC may not be capable of holding enough Ca for plant growth.
The pH is another very important variable in the soil that can provide useful information in determining the need for Ca. The pH is a measurement of hydrogen, H+, another cation found in the soil. The lower the pH, the more hydrogen is found on the CEC sites. By default, if there is a H+ on the CEC sites, there cannot be a Ca++.
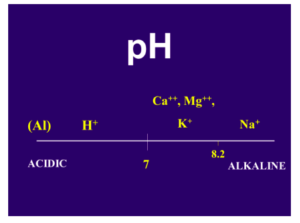
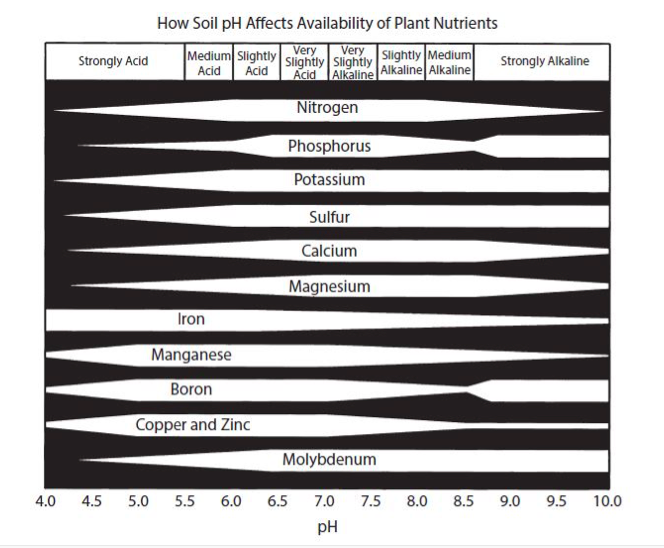
Therefore, a low-pH soil may have a deficiency of Ca and other positively charged elements, and a high-pH soil generally has an abundance of these elements (Fig. 2). The pH of the soil is a very important factor in determining the availability of the essential elements (Fig. 3). In Fig. 3, the wider the line for each element, the more readily available it is to the plant; the narrower the line, the less is available. Notice the narrowing of the line for Ca in the low (below 7) pH range. There are also good soil test procedures that can measure the amount of available Ca in the soil and can be very useful in determining the need for more Ca in the fertilization program. It is generally low-pH soils, with low CEC, that are the most likely to have plants that show a Ca deficiency.
When soil test levels for pH are very low, we have a solution. That is to “lime” the soil. Lime is calcium carbonate, CaCO3. It is readily available in most parts of the county, and is generally inexpensive. Over time, lime added to a low-pH soil will result in Ca displacing the H on the cation exchange sites, and the pH will rise. Lime is usually the best solution to a low-Ca problem, and we generally lime to bring the pH up long before Ca deficiency symptoms appear on the grass.
When someone calls with a question on Ca, the first things we want to know are the CEC and pH of the soil and the amount of available Ca in the soil. This requires a soil test. We will also ask for information on the texture of the soil – is it a sand or a clay loam?
Another important piece of the puzzle is the parent material from which the soil is formed. If it is a silica-based sand, there may well be a Ca deficiency, and supplemental applications of Ca may be required. If the parent material is CaCO3(lime), as is often the case on Midwestern and western soils in the United States, the need for more Ca is unlikely.
Because of the interest in Ca nutrition, we conducted a number of studies on exactly when Ca is needed and when it is not. A graduate student, Rodney “Rod” St. John, conducted this work. Rod completed his master’s degree and his Ph.D. on this subject. Dr. St. John now works for Ryan Lawn and Turf in Kansas City. His work was extensive, took place over a five-year period, and we will not be able to go into all of here. Fortunately, it has been published in several papers, and we would direct you there for more information (St. John et al. 2001, 2005, 2006, 20010, 2013a, 2013b).
The basic question behind the work centered on the need for calcium supplements. There were people selling Ca supplements that were telling customers that even though their root systems were growing in a soil that was high in calcium carbonate (calcareous), this calcium was not available to plants, and more was needed.
To test this, Rod grew grass on both silica-sand-based media and calcareous-sand media. Grouped within these sand types, he applied a number of calcium sources to test if they improved the turf or if they were not needed. He found that grass grown in a calcareous media did not benefit from additional Ca. The plant was getting all of the Ca it needed from the calcium carbonate in the parent material, and more did not help. Grass grown on the silica-sand did benefit from additional Ca. He also observed that the less expensive Ca sources were just as effective as the more expensive Ca supplement materials that were being marketed to the industry. In other words, if you have a Ca deficiency, good-old lime is just as effective as a very expensive Ca supplement.
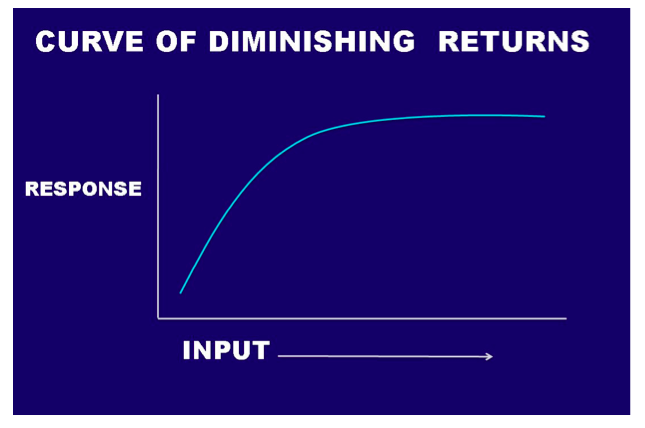
Another way to look at the question of the need for Ca is to consider a curve of diminishing returns (Fig. 4). The curve of diminishing returns simply states that where a deficiency exists, the first increments of the deficient element will produce a considerable response, whereas each additional increment produces a diminishing response until you reach the sufficient level of that material and no further response can be expected. In the field of agronomics, these curves are also known as “yield curves,” and studies are constantly underway with a variety of crops and soil types to determine where the leveling-off point occurs. It makes no sense economically to apply more than is needed for maximum yield. In turfgrass management, our goal is usually not yield of tissue, but aesthetics (how the area looks), but the concept still holds true. If you have enough of a particular element, such as Ca, more is not going to help. If the grass is growing on a media that is very low in Ca, such as a silica-based sand, the grass will likely show a deficiency of Ca. The application of an ounce or two of Ca/1,000 ft2would likely elicit a measureable response on the plant. An additional ounce would provide some response, but not as great as the first application. As more and more is added, a point is quickly reached at the top of the curve where no additional response will occur. In the calcareous media, where you already have enough Ca, more will not show any improvement in the plant, and you are wasting your money. So, does Ca work? The answer is yes if you have the conditions in which Ca is truly deficient; but the answer is no if you already have enough Ca.
In addition to the papers mentioned above, see the latest edition of “Fundamentals of Turfgrass Management 5th ed.” for more information on Ca nutrition of turf (Christians et al., 2016).
Nick Christians, Ph.D., is University professor of turfgrass management, Iowa State University, Department of Horticulture, Ames, Iowa. Adam Thoms, Ph.D., is assistant professor specializing in commercial turfgrass management, Iowa State University, Department of Horticulture, Ames, Iowa.
Sources:
Christians, N.E., A. Patton, and Q. Law. 2016. Fundamentals of Turfgrass Management 5th ed.
St. John, R.A., N.E. Christians, H.G. Taber. 2001. Supplemental Calcium applications to turfgrass established on calcareous soils. Inter. Turfgrass Soc. Res. J. 9:437-441.
St. John, R.A. 2005. Cation Ratios and Soil Testing Methods for Sand-Based Golf Course Greens. Ph.D. Dissertation, Iowa State University.
St. John, R. and N.E. Christians. 2006. Soil testing methods for sand-based putting greens. USGA Turfgrass and Environmental Research Online. July 1. 5(13): p. [1-5].
St. John, R.A and N.E. Christians. 2010. Special Approaches are needed when testing calcareous sands. Online. Applied Turfgrass Science doi:10.1094/ATS-2010-0831-01-RS.
St. John, R.A. and N.E. Christians. 2013a. Basic Cation Saturation Ratio Theory Applied to Sand Based Putting Greens. International Turfgrass Journal. 12:581-592.
St. John, R.A., N.E. Christians, H. Liu, and N.A. Menchyk. 2013b. Secondary nutrients and micronutrient fertilization. In: Stier, J.C., B.P. Horgan, S.A. Bonos, and G.K Schmitt. eds. Turfgrass: Biology, Use, and Management. Madison, Wisconsin: American Society of Agronomy. p. 521-543.